#8 The nitrogen-debate: The misleading name of the nitrogen crisis
Almost every day, we hear about human influences on global warming and climate change due to the increased greenhouse gas emissions. Examples are carbon monoxide (CO), carbon dioxide (CO2), sulfur dioxide (SO2), and now we hear a lot about nitrogen. In science, nitrogen is a non-reactive gas with the chemical formula N2. This (di)nitrogen forms about 78% of Earth’s atmosphere, and we all breathe it in without it causing us any harm. Nevertheless, we “suddenly” have a nitrogen-crisis that has a massive impact on our environment. However, the bad guy is not nitrogen, but its oxidized and reduced forms.
In traffic, industry, and our households, we produce the oxidized forms of nitrogen. At high temperatures, nitrogen and oxygen react to form nitrogen monoxide (NO) (Figure 1a) and with the addition of an extra oxygen molecule, nitrogen dioxide (NO2) (Figure 1b). They are named together as NOx.[1]
Figure 1. The reaction mechanism for the formation of nitrogen monoxide (a) and nitrogen dioxide (b).[1]
However, the agriculture sector has the most significant influence on the increase of different nitrogen-containing compounds. In the nitrogen cycle, nitrogen gas from the atmosphere and organic materials from animals and plants are converted into nutrients by bacteria and fungi in the soil. These nutrients, such as ammonia, are consumed by plants, which modify the nutritions in e.g., amino acids, nucleotides, and proteins. We and other animals are depending on these plants since we need the amino acids and nucleotides for our cells. The excess of nutritions is converted back into nitrogen gas.
At the beginning of the 20th Century, C. Bosch and F. Haber developed a procedure for the transformation of nitrogen with hydrogen into the gas ammonia (NH3) with the utilization of a metal catalyst at elevated temperatures, and high pressure (Figure 2).[2] Since then, ammonia could increasingly be utilized as fertilizer for the lands to enhance the growth of plants. This appeared to be one of the main reasons for the increase of the world population, with 1.6 billion around 1900 till almost 8 billion today.[3]
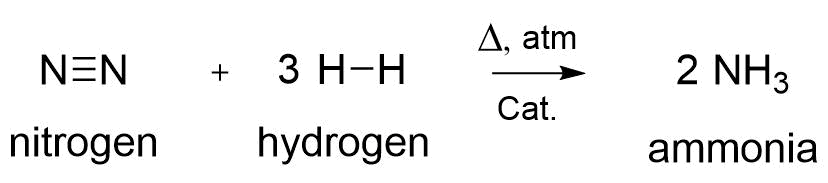
Figure 2. The reaction mechanism for the formation of ammonia.[2]
The now high demand for plants and the increased use of fertilizer disturbs the balance of the nitrogen cycle. The excess of nutrients cannot be converted back into nitrogen gas fast enough, resulting in the loss of nitrogen in various forms (e.g., NH3, NOx). Other disadvantages are the acidification of soil and the extinction of nitrogen-sensitive plants, which can result in the extinction of animal species.
References
[1] Clean air technology center (MD-12). Nitrogen Oxides (NOx), why and how they are controlled. November 1999.
[2] Bosch, C. Process of Producing Ammonia. U.S. patent 990,191, March 2, 1908.
[3] Smil, V. Nature 1999, 400, 415.
[4] College Stikstof: Jan van Maarseveen. https://www.bnnvara.nl/dewerelddraaitdoor/videos/519728 (accessed May 6th, 2020). De wereld draait door.




